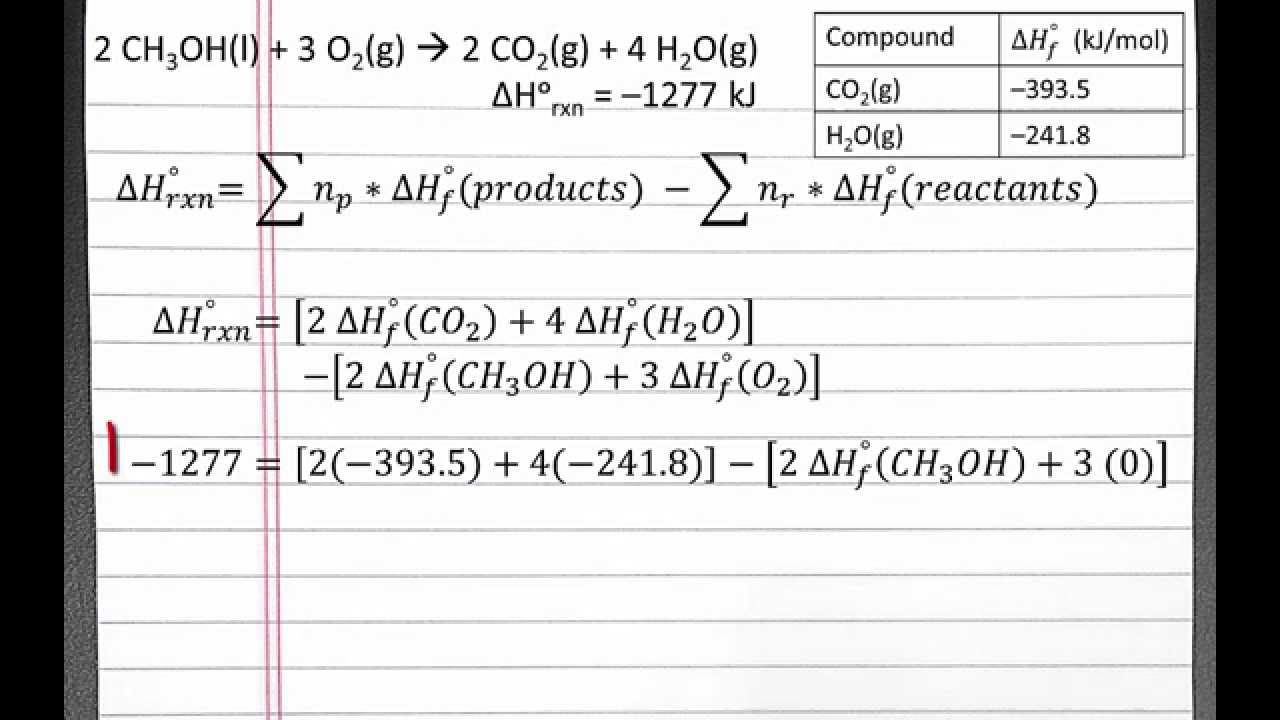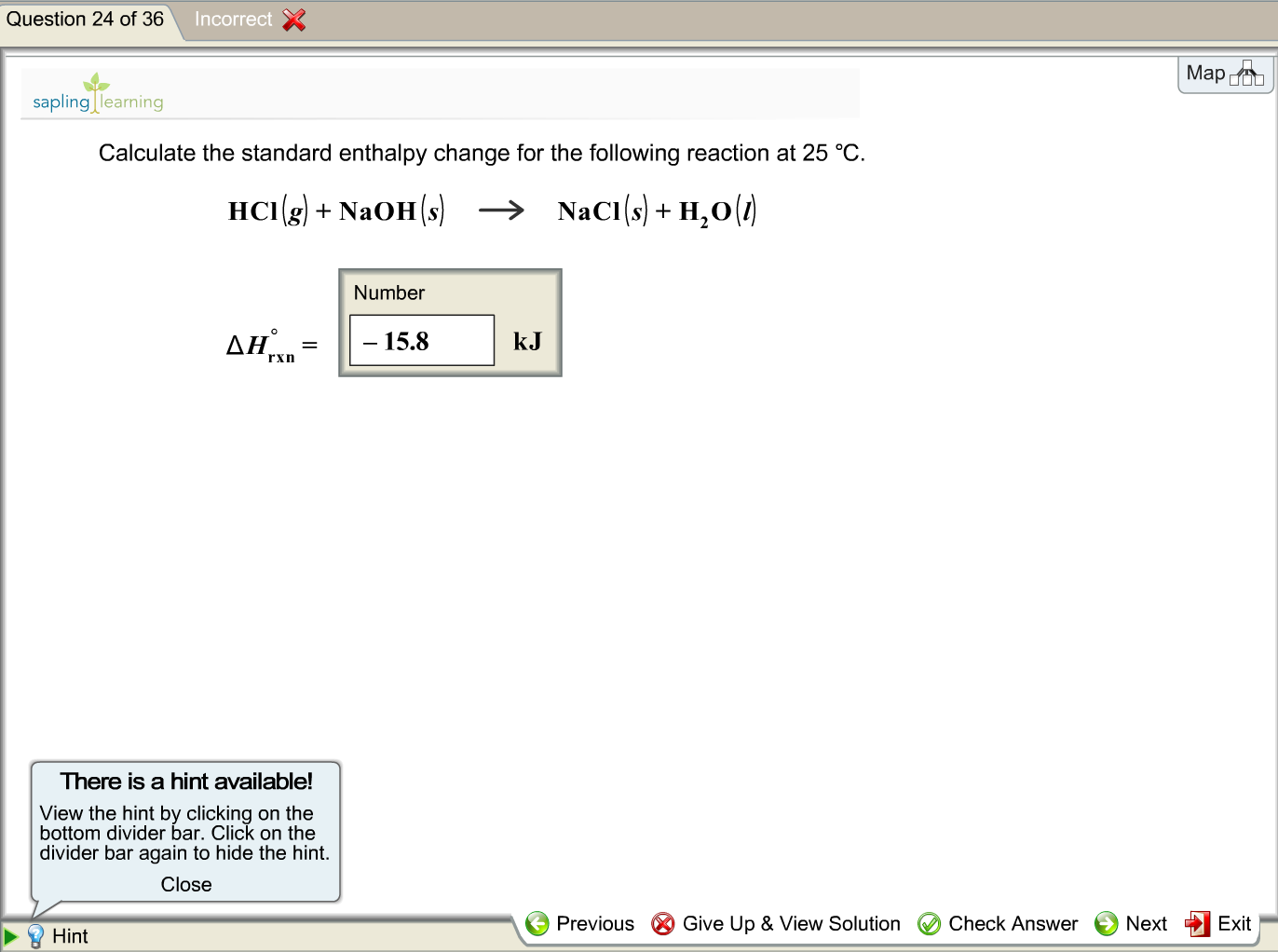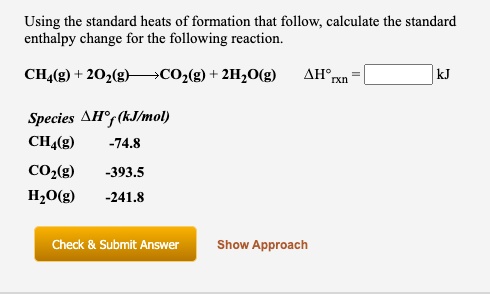
SOLVED: Using the standard heats of formation that follow; calculate the standard enthalpy change for the following reaction. CHy(g) 202(g) COz(g) 2H,O(g) AHCrxn Species AH (kJlmol) CHy(g) -74.8 COz(g) 393.5 HzO(g) 241.8

Question Video: Determining the Standard Enthalpy of Formation of Ethanol Using Standard Enthalpies of Combustion | Nagwa
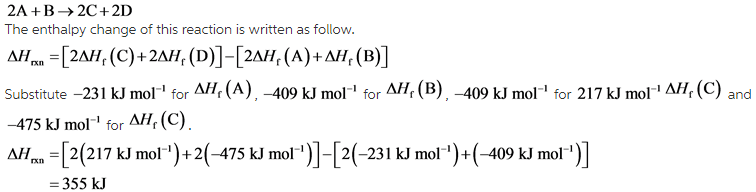
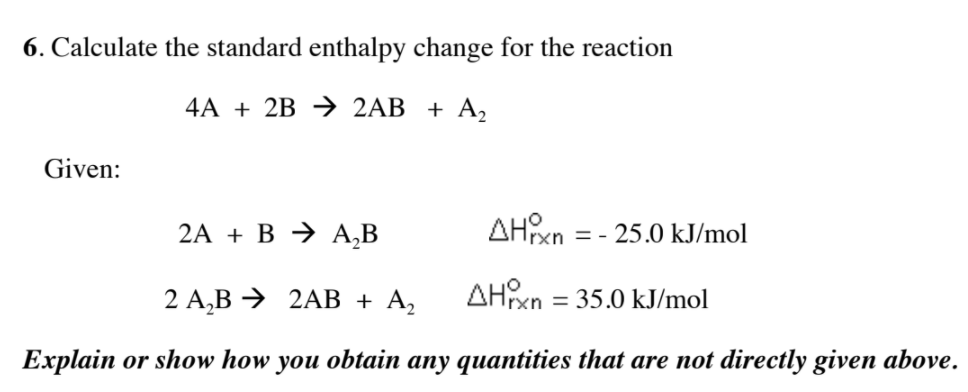
Calculate the standard enthalpy change for the reaction: 2A+B <===> 2C+2D - Home Work Help - Learn CBSE Forum

How would you calculate the standard enthalpy change for the following reaction at 25 °C: H2O (g) + C (graphite)(s) --> H2 (g) + CO (g)? | Socratic
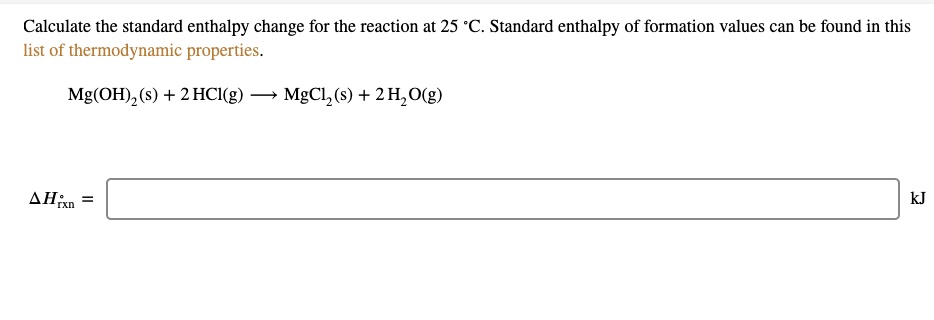
SOLVED: Calculate the standard enthalpy change for the reaction at 25 'C. Standard enthalpy of formation values can be found in this list of thermodynamic properties. Mg(OH) (s) + 2 HCl(g) MgCl,(s) +
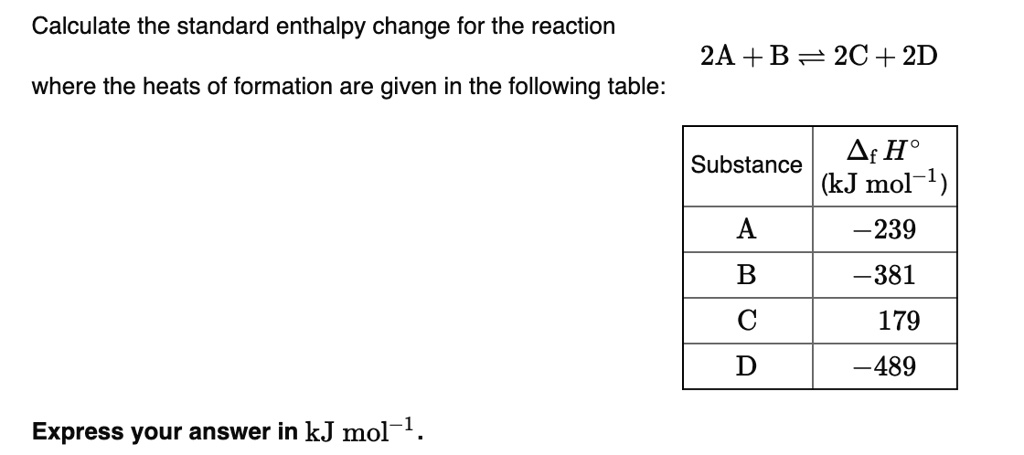
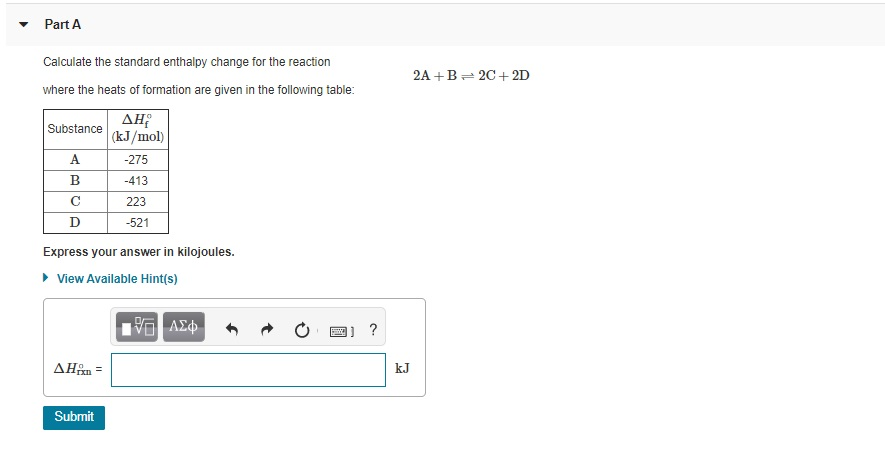
SOLVED: Calculate the standard enthalpy change for the reaction 2A +B = 2C + 2D where the heats of formation are given in the following table: Af Ho Substance (kJ mol-1) A

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (